CMS recently published the FY 2019 Inpatient Prospective Payment System (IPPS) Proposed Rule which aims to achieve greater interoperability and reduce burden so that hospitals are better able to deliver high quality care. To this end, CMS has proposed changes to the Hospital Inpatient Quality Reporting (IQR) Program and Medicare & Medicaid EHR Incentive Programs for eligible hospitals and critical access hospitals (CAH). For the complete text of the rule, see the Federal Register.
Hospital Inpatient Quality Reporting (IQR) Program
Given CMS’ goal of reducing reporting and administrative burden on providers and creating a smaller set of meaningful measures, CMS is proposing to remove several measures from the Hospital IQR Program that are also in one of the value-based purchasing programs—Hospital Value-Based Purchasing, Hospital Readmissions Reduction, and Hospital Acquired Conditions Programs. To this end, CMS is proposing a new measure removal factor, Factor 8, in that “The cost associated with a measure outweighs the benefit of its continued use in the program.” By means of the previously adopted removal factors and this proposed removal factor, CMS proposes to remove several claims-based measures, web-based measures, chart-abstracted measures, and electronic clinical quality measures (eCQMs) over the next few reporting periods.
For CY 2019, CMS is proposing that hospitals reporting to the IQR program must submit 4 self-selected eCQMs from the available set of 15 for at least one self-selected calendar quarter of CY 2019, with a submission deadline of February 29, 2020. These reporting requirements are the same as both CY 2017 and 2018 reporting. However, beginning with the CY 2020 reporting period, CMS proposes to remove 7 of the 15 available eCQMs under the new proposed removal factor. The proposed eCQMs for removal are: AMI-8a, CAC-3, ED-1, EHDI-1a, PC-01, STK-8, and STK-10. Furthermore, beginning with CY 2019 reporting period, CMS is proposing to require that hospitals participating in IQR use only 2015 Edition certified EHR technology (CEHRT). Specifically, for eCQM reporting, the 2015 Edition includes updates to standards for structured data capture and data elements, as well as features that should improve the functionality and value of eCQM data.
Regarding chart-abstracted measures in the Hospital IQR Program, CMS is proposing to remove VTE-6 and ED-1 beginning with the CY 2019 reporting period and ED-2 beginning with the CY 2020 reporting period under removal Factor 8. Should removal Factor 8 not get finalized as proposed, CMS will be unable to remove these chart-abstracted measures as well as the eCQMs. CMS also proposes to remove IMM-2 beginning with CY 2019 reporting as its measure performance is “topped out”.
Looking forward, CMS is interested in future inclusion of a Hospital-Wide, All-Cause, Risk-Standardized Mortality measure in the IQR program, but is looking for feedback as to whether to propose to adopt a Claims-Only or a Hybrid version of the measure. Additionally, CMS is also considering a new eCQM, the Hospital Harm – Opioid-Related Adverse Events eCQM, for potential future inclusion in this program.
Medicare & Medicaid EHR Incentive Programs
In this proposed rule, CMS announces its plan to overhaul the Medicare & Medicaid EHR Incentive Programs, beginning with a renaming of the program. To meet its goals of focusing on interoperability and measures that require the electronic exchange of health information between providers and patients, CMS has re-named the EHR Incentive Programs as “Promoting Interoperability (PI).”
Additionally, in this rule, CMS is proposing a new performance-based scoring methodology instead of the current threshold-based methodology beginning with CY 2019 reporting. This new scoring methodology would apply to Medicare/Dual-Eligible hospitals and CAHs attesting to the Medicare Promoting Interoperability Program; it would not apply to Medicaid only hospitals, although CMS is proposing that States could choose to adopt this methodology. Under this proposed scoring methodology, CMS is proposing a smaller set of objectives which would include e-Prescribing, Health Information Exchange, Provider to Patient Exchange, and Public Health and Clinical Data Exchange and a reduction in the number of required measures from 16 to 6. Hospitals would be required to report certain measures from each of the four objectives, with performance-based scoring happening on the individual measure-level. The scores for each individual measure would be added together to calculate the total Promoting Interoperability score of up to 100 points. Hospitals scoring below 50 points would not be considered a meaningful user.
The proposed performance-based scoring methodology for CY 2019 reporting is outlined below:
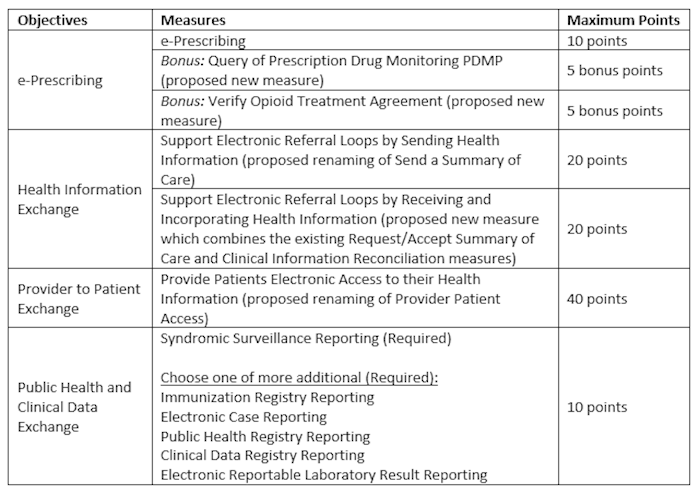
To earn any score in the PI Program, CMS is proposing that hospitals would have to attest that they completed a Security Risk Analysis (Protect Patient Health Information objective) during the calendar year in which the EHR reporting period occurs. Furthermore, CMS has proposed ways to re-distribute points should a hospital qualify for a measure exclusion. All other Stage 3 objectives or measures are proposed for removal.
Should this new scoring methodology not get finalized as proposed, the hospital PI Program would maintain the current Stage 3 methodology with the same objectives, measures and thresholds. However, if CMS finalizes the two new measures, Verify Opioid Treatment Agreement and Query of PDMP, these measures with a corresponding threshold of at least 1 patient will fall under the e-Prescribing objective and hospitals will have to report all 3 measures, but only meet the threshold for 1.
Beginning with CY 2019 reporting period, CMS requires that all hospitals attesting to these programs use EHR technology certified to the 2015 Edition. CMS proposes that for CY 2019 and CY 2020, new and returning eligible hospitals and CAHs will be required to attest to objectives and measures for a minimum of any continuous 90 days during the respective calendar year. For CY 2019 electronic reporting of clinical quality measures (CQMs), CMS is proposing that hospitals and CAHs must report 4 self-selected CQMs from the available 16 for at least one self-selected calendar quarter of CY 2019, with a submission deadline of February 29, 2020. These reporting requirements are the same as both CY 2017 and 2018 reporting and align with the IQR eCQM reporting requirements. Furthermore, using the same proposed removal factor as mentioned above CMS proposes to remove 8 of the 16 eCQMs beginning with the CY 2020 reporting period. The proposed eCQMs for removal are: AMI-8a, CAC-3, ED-1, EHDI-1a, PC-01, STK-8, STK-10 and ED-3. By proposing to also remove ED-3, an outpatient eCQM, CMS completely aligns the eCQMs between the IQR Program and the Promoting Interoperability Programs for hospitals. Additionally, CMS is also considering the same new eCQM as for IQR, the Opioid Harm eCQM.
With these proposals, CMS strives to reduce burden and create more meaningful programs and measures for hospitals and providers, and we will see what is finalized in the coming months. Should you have questions about your hospital reporting of eCQMs, objective measures, or chart-abstracted measures, please contact us.

