CMS recently published the FY 2020 proposed rule for the Inpatient Prospective Payment Systems (IPPS) which continue to focus on transforming the healthcare delivery system to provide patients with better care. To this end, CMS has proposed changes to the Hospital Inpatient Quality Reporting (IQR) program and Medicare & Medicaid Promoting Interoperability (PI) program for eligible hospitals and critical access hospitals (CAHs). (For the full text of the rule, see the Federal Register.)
Hospital Inpatient Quality Reporting (IQR) Program
For the Hospital inpatient Quality reporting (IQR) program, CMS proposes some changes to the set of measures. Specifically, CMS proposes adopting two new opioid-related electronic clinical quality measures (eCQMs) beginning with the CY 2021 reporting period (FY2023 payment determination): (1) Safe Use of Opioids – Concurrent Prescribing and (2) Hospital Harm – Opioid-Related Adverse Events. Additionally, beginning with the July 1, 2023 through June 30, 2024 reporting period for the FY 2026 payment determination, CMS proposes to remove the Claims-Based Hospital Wide All-Cause Readmission measure and replace it with the Hybrid Hospital-Wide-All-Cause Readmission (Hybrid HWR) Measure which includes both claims and electronic health record (EHR) data. Prior to this, CMS is proposing voluntary reporting periods for the Hybrid HWR measure which run from July 1, 2021 through June 30, 2022 and July 1, 2022 through June 30, 2023. These voluntary reporting periods would not impact payment nor be publicly reported, but the hospital will receive a confidential hospital-specific feedback report. After the end of the proposed voluntary reporting periods, CMS is proposing the Hybrid HWR measure results would be publicly reported beginning with the data collected in CY 2023 impacting FY 2026 payment determination.
The rule also includes proposals regarding the reporting of eCQMs for upcoming reporting years. For the CY 2020 reporting period (FY 2022 payment determination) and CY 2021 reporting period (FY2023 payment determination), CMS proposes that hospitals will continue to submit one, self-selected calendar quarter of data for four self-selected eCQMs in the hospital IQR program measure set. However, for the CY 2022 reporting period (FY2024 payment determination), CMS proposes that hospitals will be required to report one, self-selected calendar quarter of data for four eCQMs where three are self-selected eCQMs and one is the proposed Safe Use of Opioids – Concurrent Prescribing eCQM. EHR technology must continue to be certified to all eCQMs available to report for the CY 2020 reporting period and subsequent years.
CMS is also looking for public comment on three potential new eCQMs for the IQR program: (1) Hospital Harm – Severe Hypoglycemia, (2) Hospital Harm – Pressure Injury, and (3) Cesarean Birth.
Medicare & Medicaid Promoting Interoperability Programs
CMS continues to propose changes to the Medicare and Medicaid promoting interoperability (PI) programs with the aim to reduce administrative burden, continue the use of 2015 Edition CEHRT, and improve patient access to their EHRs so they can make fully informed health care decisions.
CMS is proposing that new and returning hospitals participating in the Medicare Promoting Interoperability (PI) Program will report to CMS a minimum of any continuous 90-day period in CY 2021. When CMS renamed the EHR Incentive Program to Promoting Interoperability and finalized a new scoring methodology, they did not state whether the actions in the numerator are limited to the EHR reporting period or whether they would allow actions in the numerator to count outside of the EHR reporting period but within the calendar year. Thus, CMS is proposing that beginning with CY 2020 hospitals under the Medicare PI Program would attest to numerators and denominators of PI measures that include only actions that have occurred during the EHR reporting period selected by the hospital. Currently all Medicare PI measures except for the measures associated with the Public Health and Clinical Data Exchange Objective contain this numerator limitation in the measure specification. The one exception to this proposal is the Security Risk Analysis measure, which may occur any time during the calendar year in which the EHR reporting period occurs. This proposal does not apply to the Medicaid PI Program for eligible hospitals or eligible professionals, as these programs include measures that were removed only from the Medicare PI Program. Among these Medicaid PI measures, CMS will continue to allow, but not require the inclusion of numerator actions outside of the reporting period.
While the Query of PDMP measure was finalized as a required measure for CY 2020 reporting, CMS is proposing to change this measure to optional and available for 5 bonus points. CMS would remove the CY 2020 exclusion for this measure as it would be optional to report. Furthermore, for CY 2019 reporting, CMS is proposing changing this measure from a numerator/denominator requirement to a yes/no attestation, in which a “yes” response would indicate that for at least one Schedule II opioid electronically prescribed using CEHRT during the reporting period, the hospital used data from CEHRT to conduct a query of a PDMP for prescription drug history. Beginning with the CY 2020 reporting period, CMS is proposing to remove the Verify Opioid Agreement measure from the PI Program.
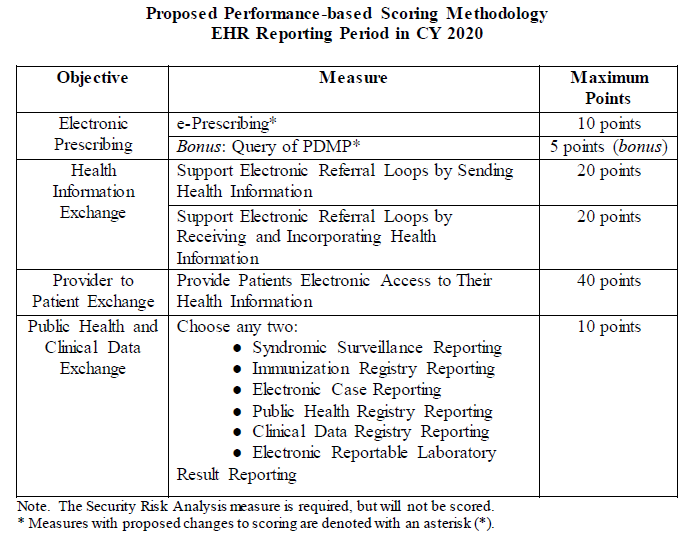
CMS plans to continue to align the CQM reporting requirements for the PI Programs with those for the Hospital IQR Program. In line with the proposal of adding the two opioid CQMs to IQR, CMS is also proposing these two measures for PI— (1) Safe Use of Opioids – Concurrent Prescribing and (2) Hospital Harm – Opioid-Related Adverse Events—beginning with CY 2021 reporting. Further, CMS is seeking comments on whether they should consider proposing to adopt the Hybrid HWR Measure with claims and EHR data in future rulemaking starting with the CY 2023 reporting period (July 1, 2023 through June 30, 2024).
To reduce burden for hospitals, CMS is proposing to align the reporting period requirements for PI with IQR. To this end, hospitals will continue to submit one, self-selected calendar quarter of data for four self-selected eCQMs in the PI Program measure set for the CY 2020 reporting period and CY 2021 reporting period. However, for the CY 2022 reporting period (FY2024 payment determination), hospitals will be required to report one, self-selected calendar quarter of data for four eCQMs where three are self-selected eCQMs and one is the proposed Safe Use of Opioids – Concurrent Prescribing eCQM. Hospitals that must submit to CMS by attestation where electronic reporting is not feasible will have to report on all CQMs in the PI measure set for the full calendar year (consisting of 4 quarterly data reporting periods). Though, beginning with CY 2023 reporting, CMS is proposing to eliminate attestation as a method for reporting CQMs for Medicare PI Program and instead require all hospitals to submit their CQM data electronically. EHR Technology must continue to be certified to all eCQMs available to report for the CY 2020 reporting period and subsequent years.
Looking to the future of the PI Program, CMS is seeking suggestions on more meaningful opioid measures to include in the program. CMS is also interested in hearing feedback about including Medicare PI Program data on the Hospital Compare website, integration of Patient-Generated Health Data into EHRs using CEHRT, activities that promote the safety of the EHR, and measures requiring the use of an API.

